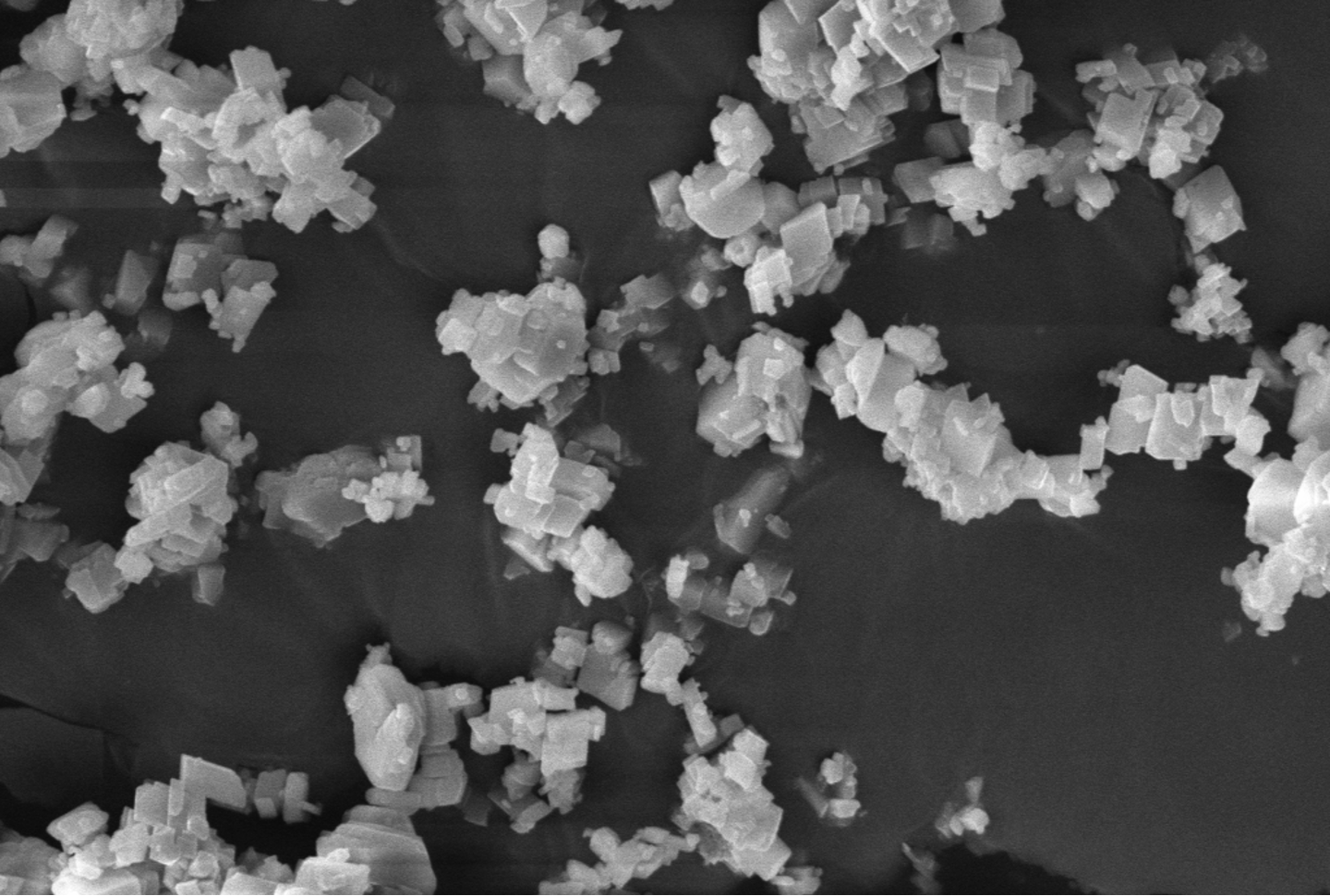
Cement production accounts for 8% of all man-made CO2 emissions. This is largely due to the use of carbon-containing limestone (CaCO3) as a key feedstock material. The calcination of limestone to obtain lime (CaO) leads to direct CO2 emissions, while the use of fossil fuels for heating leads to indirect CO2 emissions. In order to reduce the carbon footprint of concrete, waste utilization and carbon mineralization are promising approaches, where industrial wastes can be used as alternative carbon-free feedstock and carbon mineralization can offer permanent storage of industrially emitted CO2.
We are investigating the use of carbonates and silicates as fillers and supplementary cementitious materials to replace carbon-intensive Portland cement, where the solid carbonates and silicates are derived from industrial wastes through innovative extraction and carbon mineralization techniques. In a separate project, we are exploring the use of waste-to-energy ash as fine and coarse aggregate replacement, to provide a safe and permanent use of harmful waste that would otherwise need to be landfilled. And we are also looking at the potential to use Mg derived from seawater through innovative electrochemical processes to produce Mg binders that harden through carbonation curing.
Relevant publications:
- Rim, G., Roy, N., Zhao, D., Kawashima, S., Stallworth, P., Greenbaum, S. G., & Park, A. H. A. (2021). CO 2 utilization in built environment via the P CO2 swing carbonation of alkaline solid wastes with different mineralogy. Faraday Discussions. doi:10.1039/D1FD00022E
- Tian, Y., Bourtsalas, A. T., Kawashima, S., Ma, S., & Themelis, N. J. (2020). Performance of structural concrete using Waste-to-Energy (WTE) combined ash. Waste Management, 118, 180-189. doi:10.1016/j.wasman.2020.08.016
- Badjatya, P., Akca, A., Alvarez, D.F., Chang, B., Ma, S., Pang, X., Wang, E., van Hinsberg, Q., Esposito, D. and Kawashima, S. (2021). Carbon-Negative Cement Manufacturing from Seawater-Derived Magnesium Feedstocks. ChemRxiv [Preprint] doi:10.33774/chemrxiv-2021-5dznt-v2